Assessment Of Risk Usp 800 Template
Hazardous drug assessment of risk aor template portions of this information and these forms are proprietary to and subject to copyright ownership of clinical iq llc and have been modified by sample pharmacy under license and for limited use.
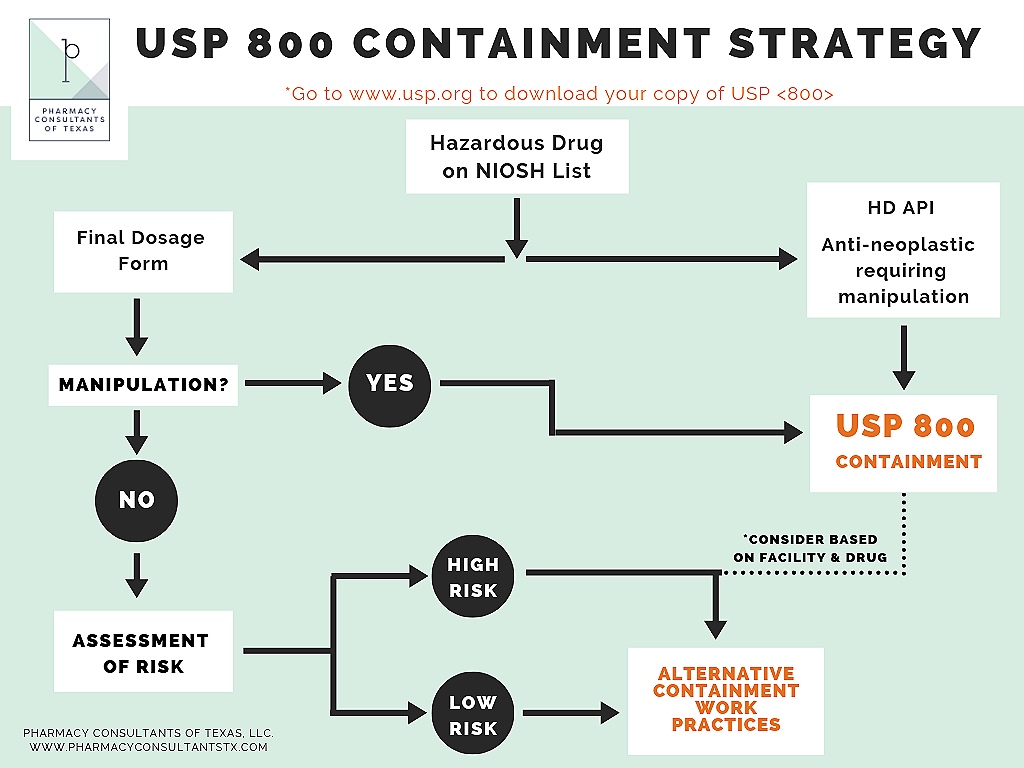
Assessment of risk usp 800 template. Per usp 800 the assessment of risk aor allows for a pharmacy to provide alternative containment strategies. A set of excel based risk assessment templates which can assist the user in this critical step of performing the risk assessment required by 800. Identify all of the hds handled within the entity including each dosage form used. Identify the drugs and dosage forms eligible for an assessment of risk design an assessment of risk to be used at your organization list the facility and monitoring elements for compliance with usp 800 prioritize gaps in compliance that need to be addressed within your organization preparation read assessment of risk section from usp 800.
What must follow usp 800 containment requirements. Ncpa has developed a blank usp 800 risk assessment template and a sample template for testosterone to help you create your own risk assessments for each hazardous drug as required by usp 800. Usp general chapter 800s implementation date is coming up on dec. Some will require full containment others can be handled with gloves alone.
Pharmecology offers a unique assessment of risk customized to your inventory as required by usp 800 and also provides a one stop solution to help your organization identify segregate manage and dispose of pharmaceutical waste in a compliant and cost effective manner. All of these resources are available as follows. Usp general chapter 800s implementation date is coming up on dec. The exposure risk determines how that drug will be handled throughout each step in the pharmacy.
In the pursuit of usp 800 compliance the first step is to identify all of the hazardous drugs hds utilized by the entity as well as their dosage forms and the specific handling. O n july 1 2018 usp 800 hazardous drugs handling in healthcare settings 1 will become official although some states accreditation organizations and facility policies may require earlier compliance. Usp 800 perform an assessment of risk to comply with usp 800 article pdf available in drug development and industrial pharmacy 143 march 2017 with 1296 reads how we measure reads. As the hdcs is organized in parallel to the usp 800 preparation checklist users can rapidly refer to it for help completing the checklist.
This review must encompass formulary items as well as any non formulary items utilized.