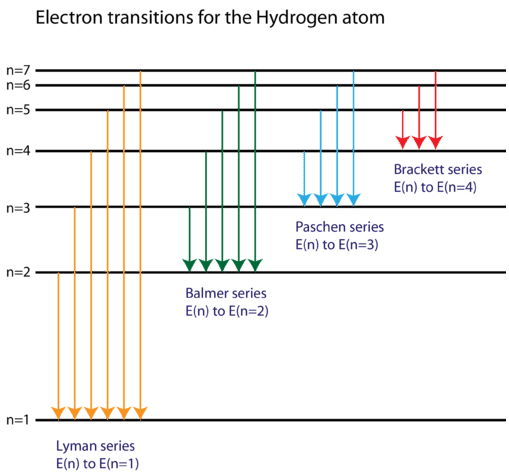
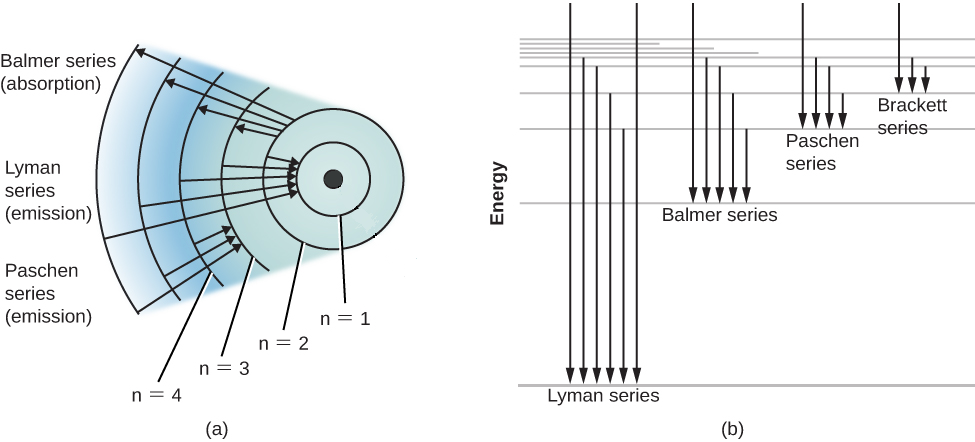
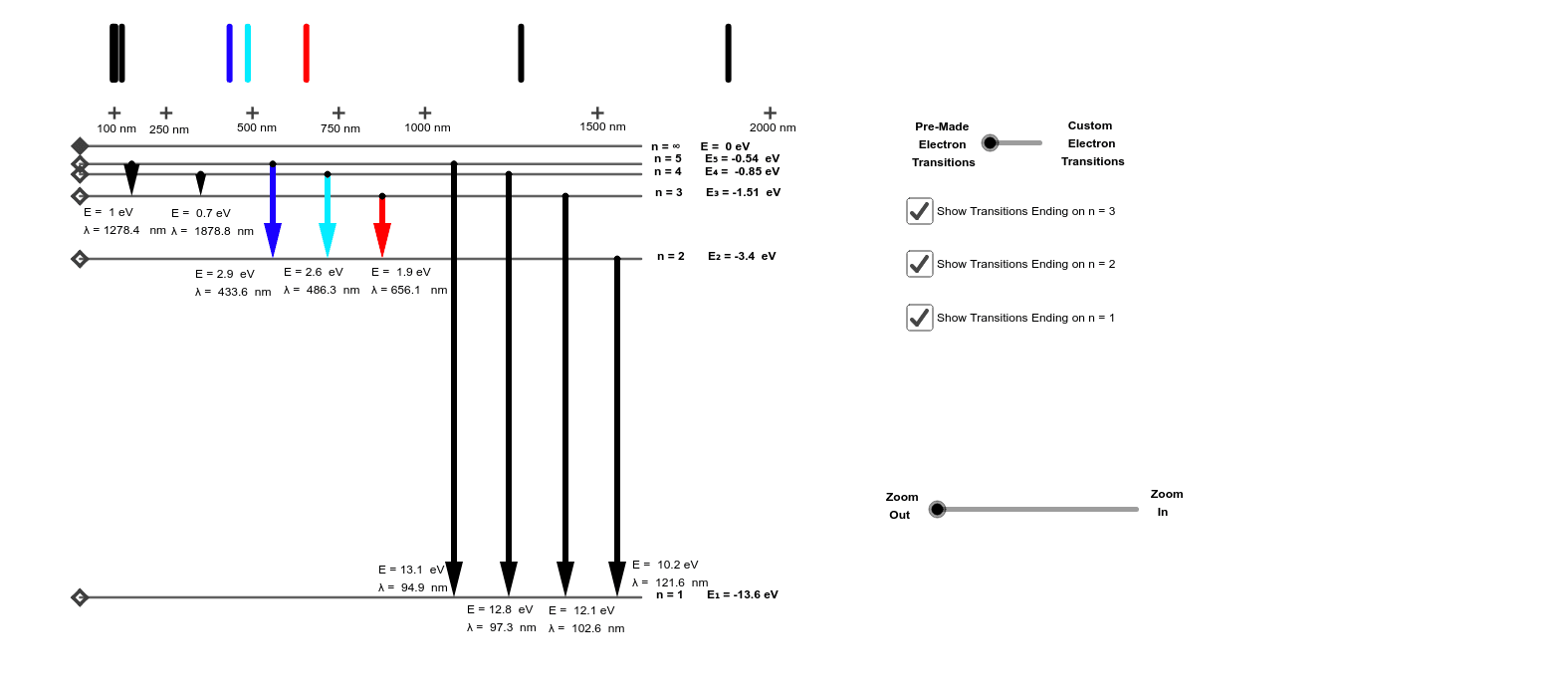
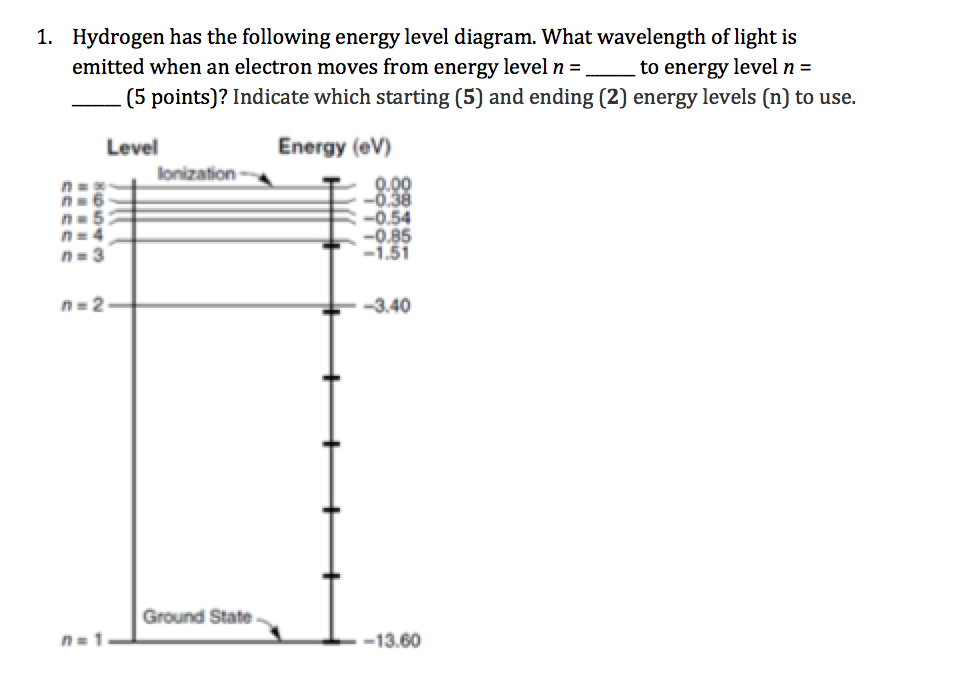
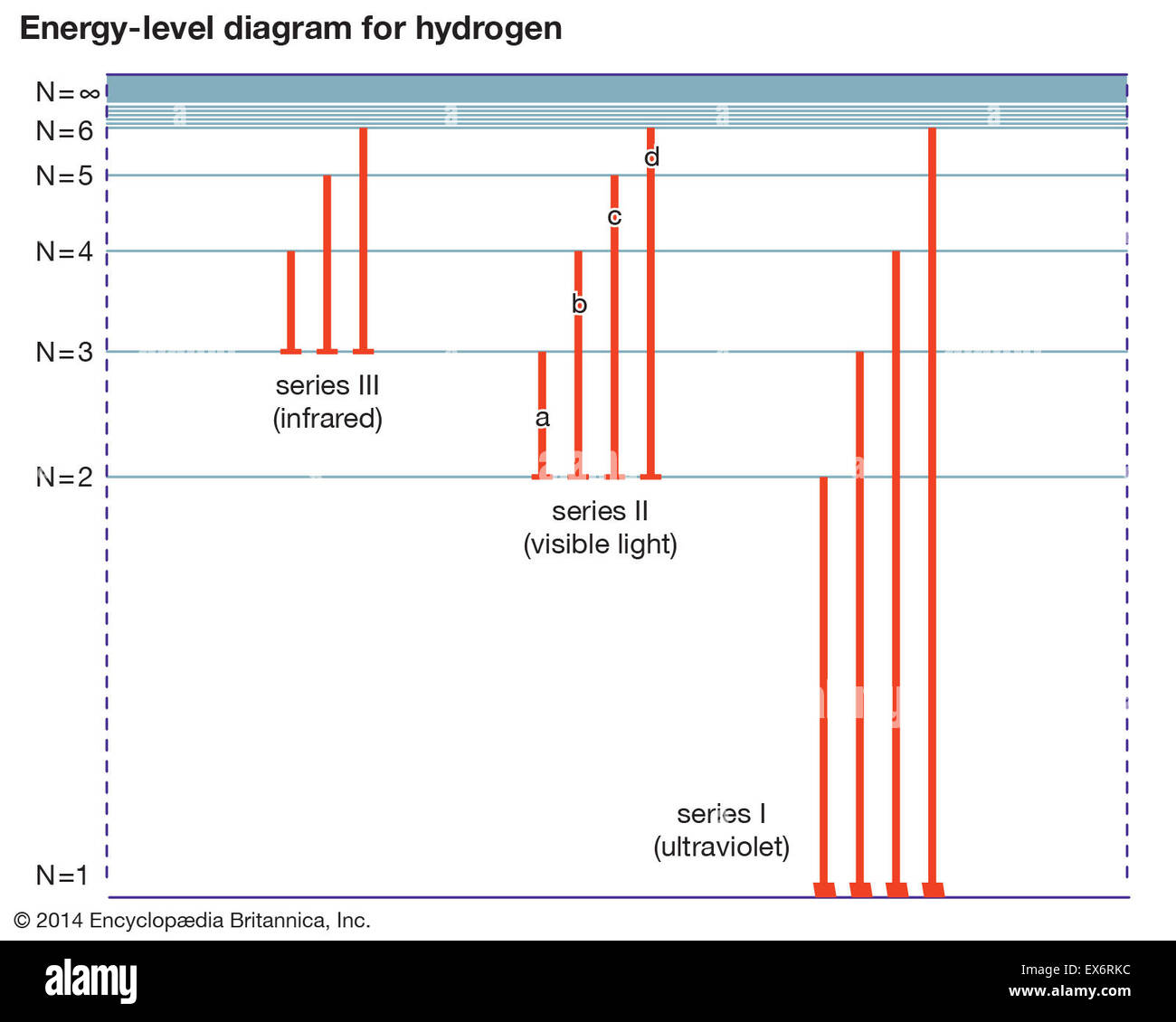
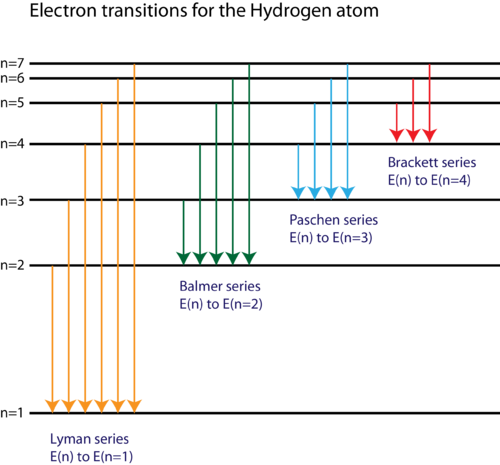
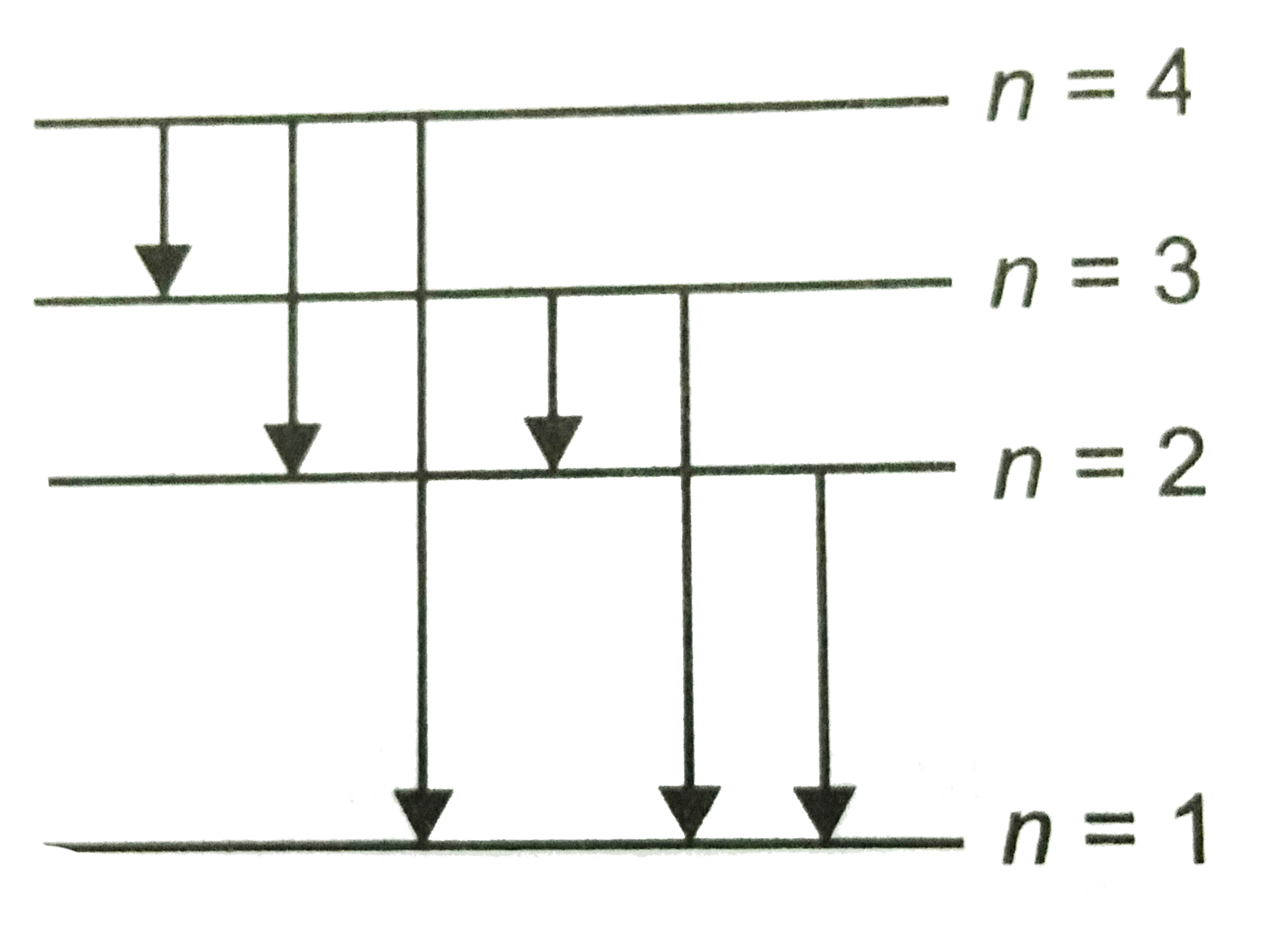
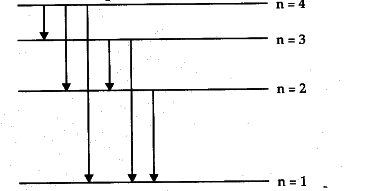
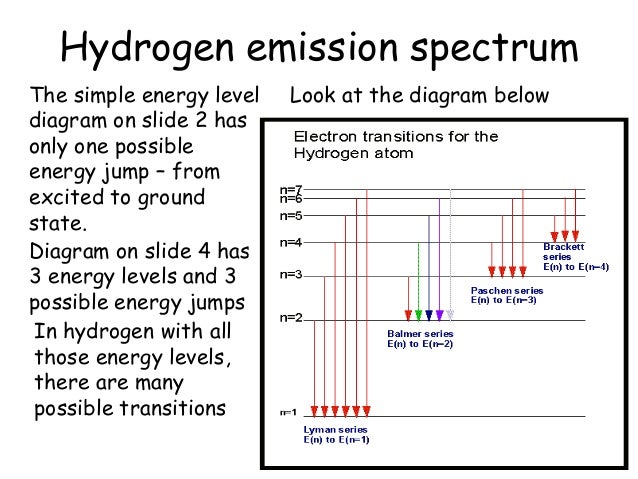
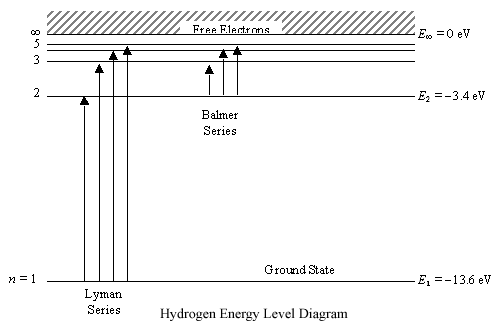
Energy Level Diagram For Hydrogen

The energy levels agree with the earlier bohr model and agree with experiment within a small fraction of an electron volt.
Energy level diagram for hydrogen. Chemists use the energy level diagram as well as electron configuration notation to represent which energy level. The energy is expressed as a negative number because it takes that much energy to unbind ionize the electron from the nucleus. In 1913 niels bohr obtained the energy levels and spectral frequencies of the hydrogen atom after making a number of simple assumptions in order to correct the failed classical model. The formula defining the energy levels of a hydrogen atom are given by the equation.
E e 0 n 2 where e 0 136 ev 1 ev 160210 19 joules and n 123 and so on. An energy level diagram is more useful and easier to work with than quantum numbers in the quantum mechanical model. This video also explains what is meant by the lyman balmer and. In practice electrons with high n eg.
The ionization energy of an atom is the energy required to remove the electron completely from the atomtransition from ground state n 0 to infinity n. Energy level diagrams and the hydrogen atom. Emission spectrum of hydrogen. The diagram for hydrogen is shown above.
Bohr model energy levels derivation using physics bohr model energy levels. Forms of such diagrams are called grotrian diagrams or term diagrams in various parts of the literature. The n 1 state is known as the ground state while higher n states are known as excited states. 100 or more are so weakly bound that even weak disturbances will pull the electron away.
For hydrogen the ionization energy 136ev when an excited electron returns to a lower level it loses an exact amount of energy by emitting a photon. How bohrs model of hydrogen explains atomic emission spectra if youre seeing this message it means were having trouble loading external resources on our website. Its often helpful to draw a diagram showing the energy levels for the particular element youre interested in. While the energy level diagram of hydrogen with its single electron is straightforward things become much more complicated with multi electron atoms because of the interactions of the electrons with each other.
Chemists sometimes use an energy level diagram to represent electrons when theyre looking at chemical reactions and bonding. The electron transition energy formula equation helps you to calculate the energy levels of electrons in the hydrogen atom only. The different energy levels of hydrogen are denoted by the quantum number n where n varies from 1 for the ground state the lowest energy level to corresponding to unbound electrons. Electrons can only be in certain discrete circular orbits or stationary states thereby having a discrete set of possible radii and energies.