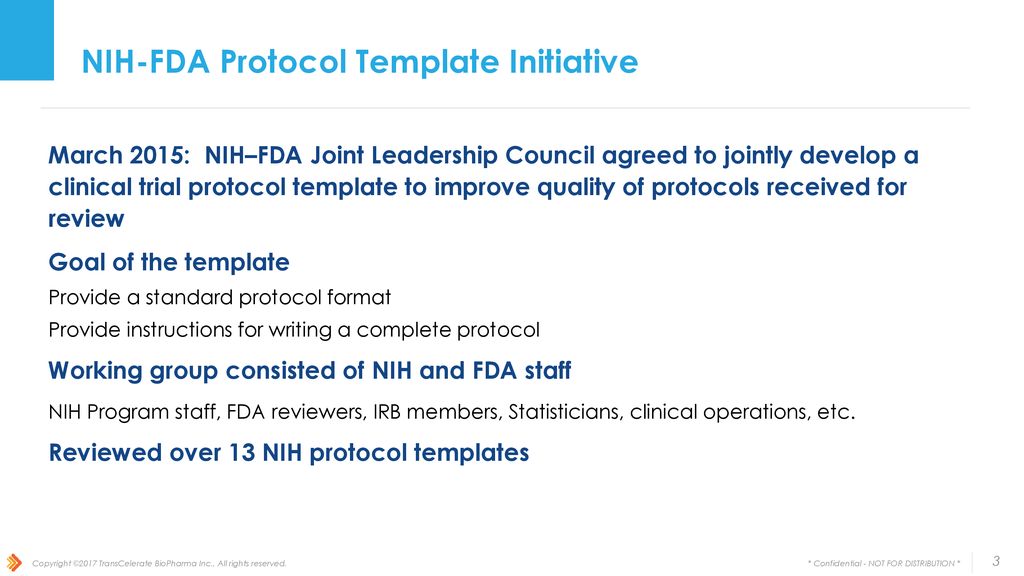
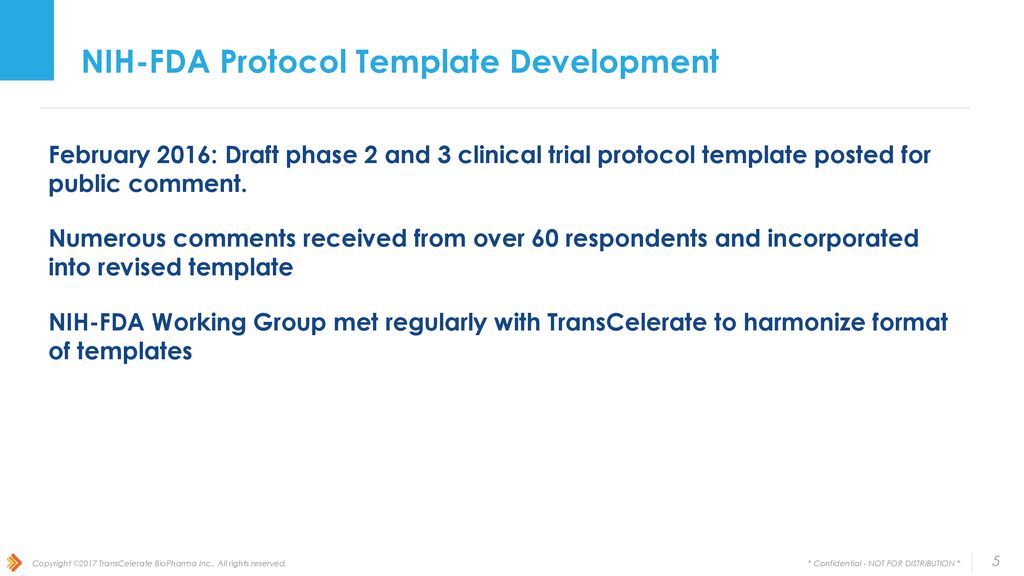
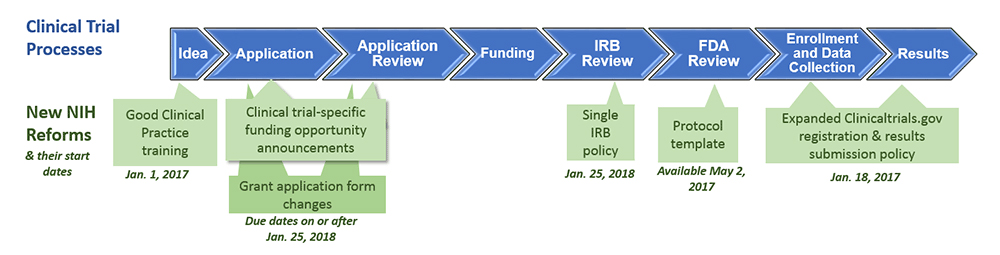
Fda Clinical Trial Protocol Template

This clinical trial protocol template is a suggested format for phase 2 and 3 clinical trials funded by the.
Fda clinical trial protocol template. Search for fda guidance documents. Before finalizing and distributing the clinical trial protocol. Nih fda clinical trial protocol template v1027 aug 20178 7 since this discovery toyos and colleagues have used ipl off label to treat hundreds of rosacea subjects presenting with dry eye disease. This protocol template aims to facilitate the development of two types of clinical trials involving human participants.
E3 structure and content of clinical study reports. The first type of trials are phase 2 and 3 clinical trial protocols that require a food and drug administration fda investigational new drug ind or. The template follows the international conference on harmonisation ich e6 r2 good clinical practice and is available as a word document. Nih fda clinical trial protocol template v10 7 apr 2017 a.
A case report from 2002 demonstrated the potential of ipl technology. Nih fda phase 2 and 3 indide clinical trial protocol template. As with all guidance documents they do not. This clinical trial protocol template is a suggested format for phase 2 or 3 clinical trials supported by the national institutes of health nih that are being conducted under a food and drug administration.
The template contains instructional and sample text for nih funded investigators to use when writing protocols. Nd preface remove this preface before finalizing and distributing the clinical trial protocol. Phase 2 and 3 clinical trials that require fda ind or ide application. Guidance documents accessible from this page represent the agencys current thinking on good clinical practice gcp and the conduct of clinical trials.
Suggested templates for phase 1 and 2 clinical trials. Generic protocol documents and instructions for ctep studies instructions for submitting protocol documents to ctep pdf step by step guide for submitting esubmission ready documents to ctep pdf generic protocol template ms word updated september 17 2019. The fda and nih are requesting public comment on a draft clinical trial protocol template that has been released for phase 2 and phase 3 ind investigational new drug investigational device exemption studies. The objective of this guideline is to facilitate the compilation of a single core clinical study.