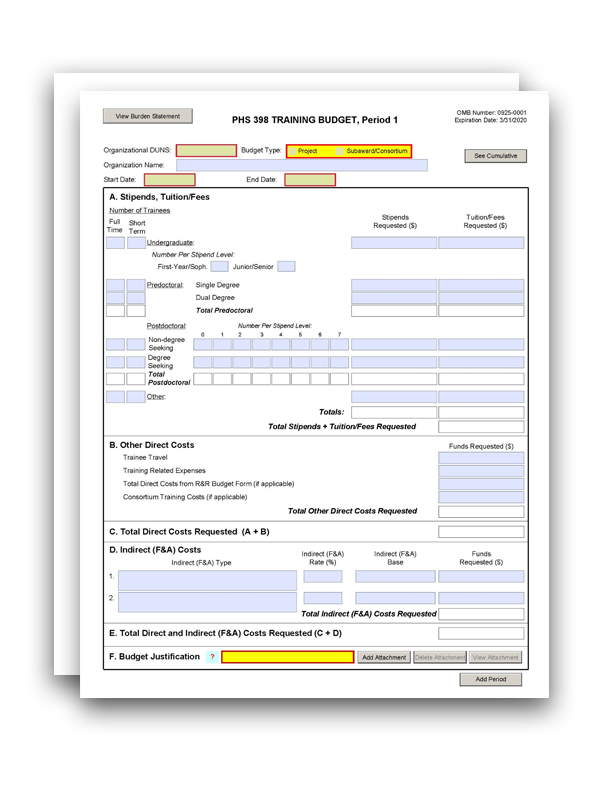
Nih Clinical Trial Budget Template

Use of this template is optional.
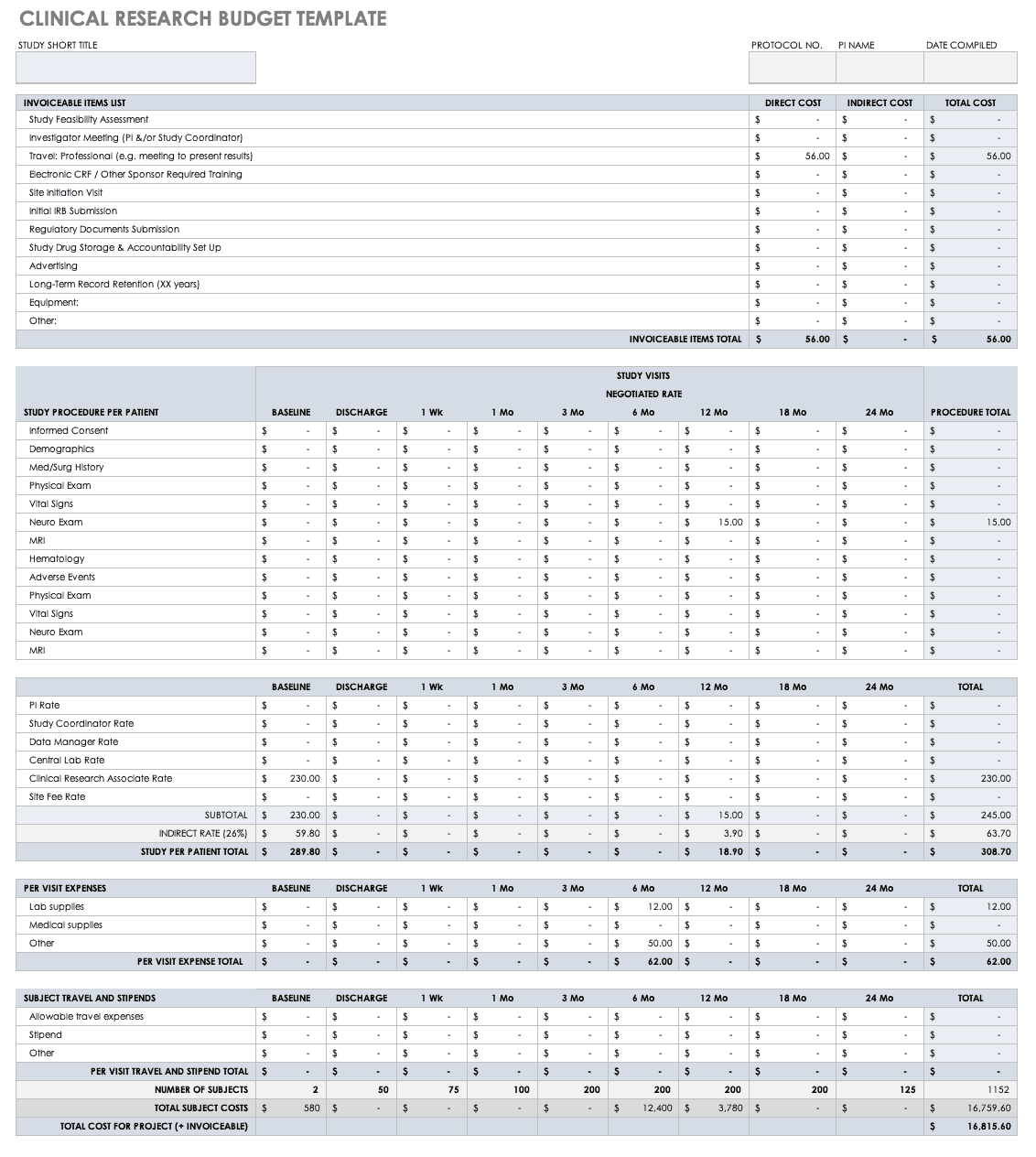
Nih clinical trial budget template. Clinical research budget template. Site budget you are the local site pi and are negotiating with industry or other funding source regarding how much you get paid for startup of trialstudy per patient enrollment and study closeout. There is a new form for consolidated human subjects inclusion enrollment report and clinical trial information. Download free clinical trial templates for your clinical research available in sharepoint word.
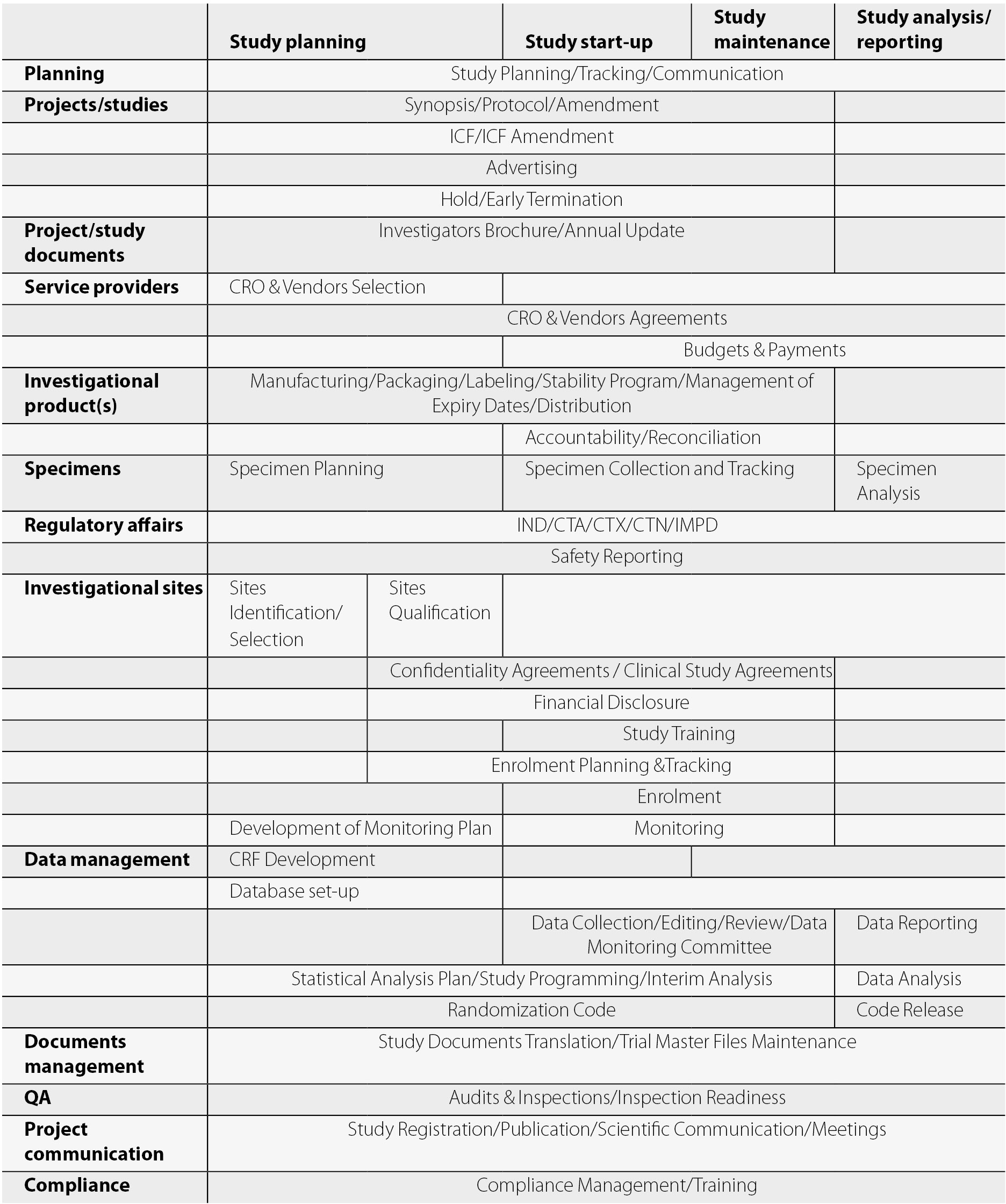
Investigator initiated clinical research data and safety monitoring guidelines and policies clinical study templates and forms nih and other federal guidelinespolicies for clinical research. Funded by the national institutes of health nih promis can be used in clinical trials as measures of conditions and disease and as a comparison. This clinical trial protocol template is a suggested format for phase 2 and 3 clinical trials funded by nih that are being conducted under a fda ind or ide application. This protocol template aims to facilitate the development of two types of clinical trials involving human participants.
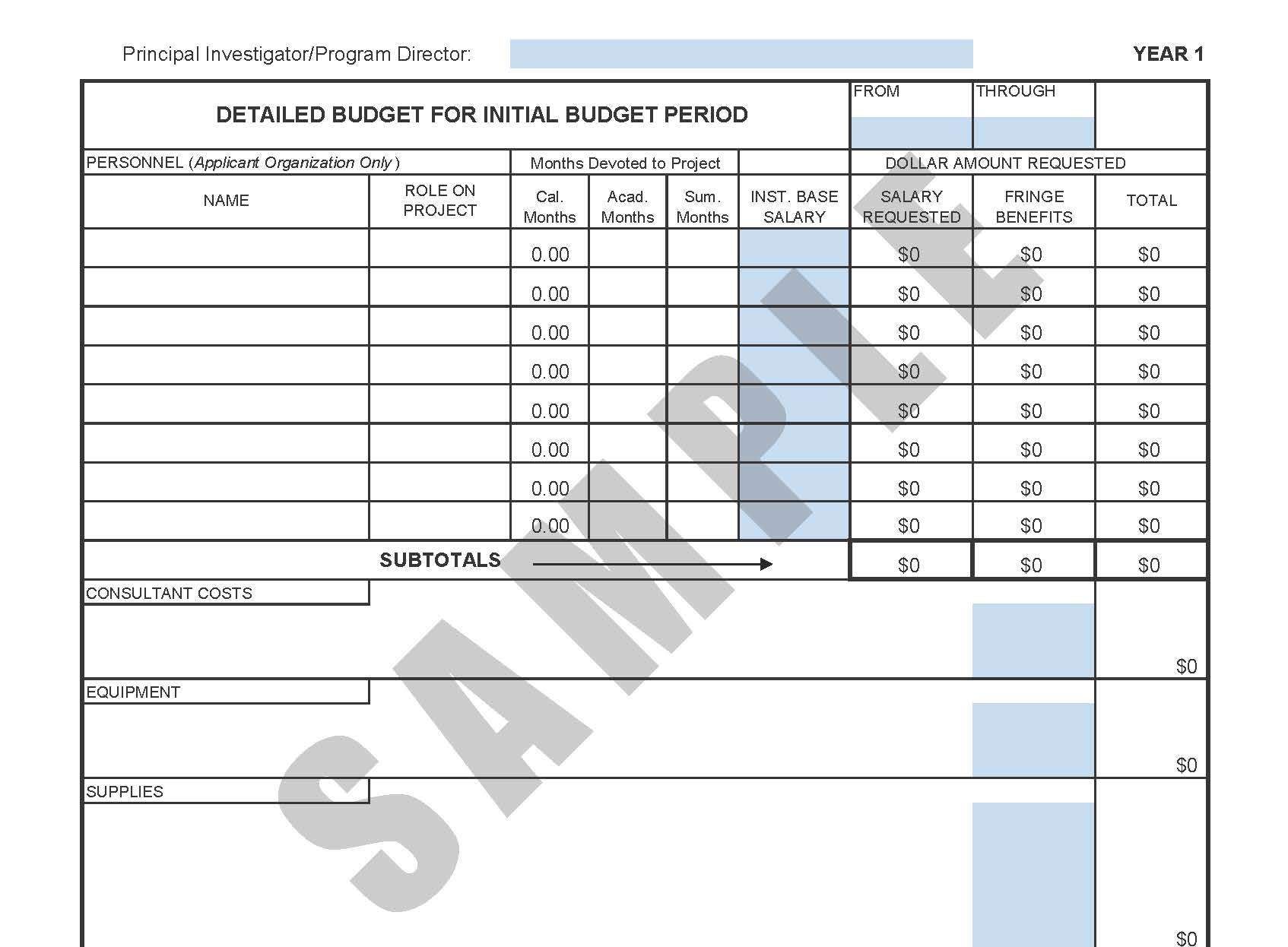
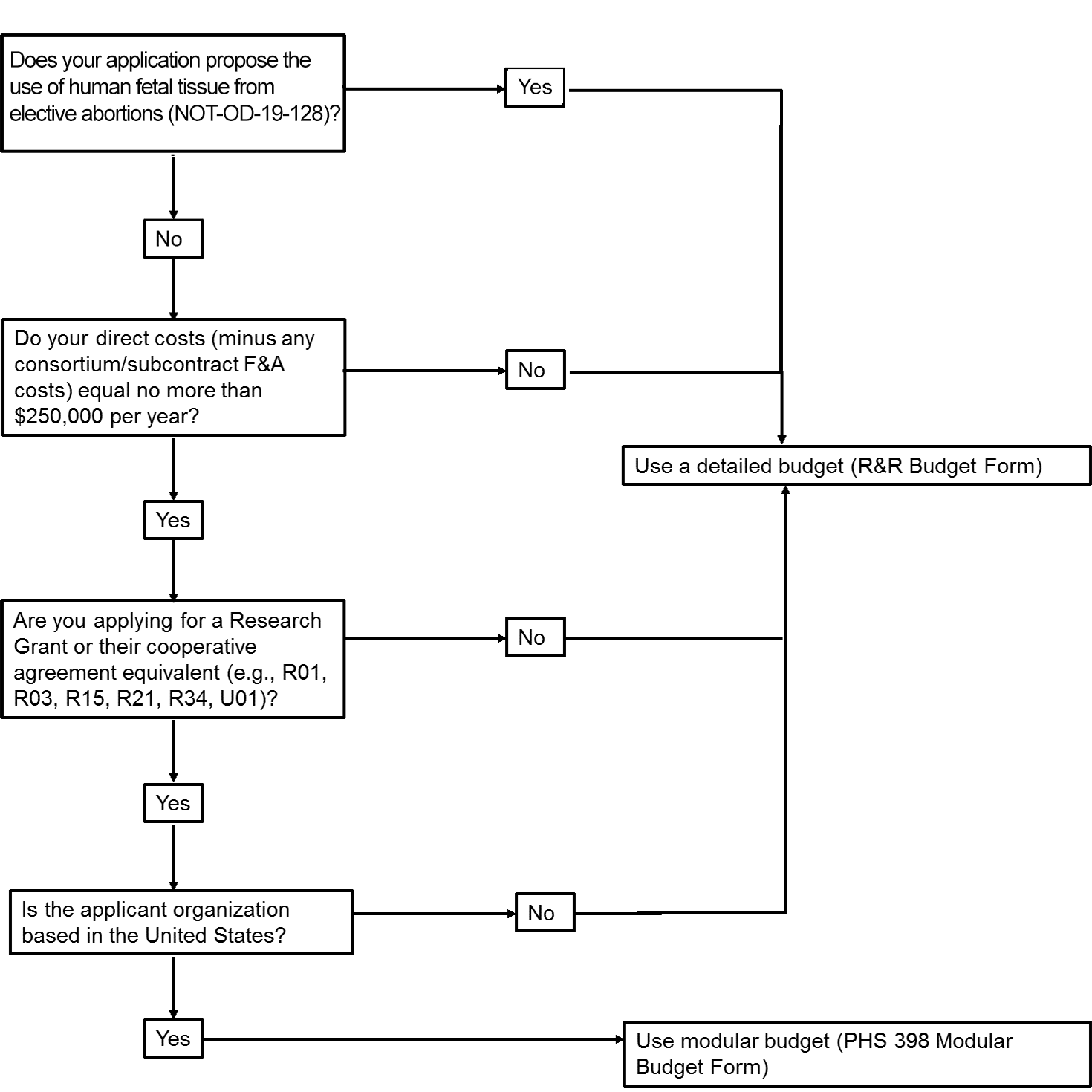
Applicants conducting phase 2 or 3 clinical trials that require investigational new drug applications ind or investigational device exemption ide applications can use an nih fda template with instructional and sample text to help write protocols. The first type of trials are phase 2 and 3 clinical trial protocols that require a food and drug administration fda investigational new drug ind or investigational device exemption ide application. Chances are youve created a budget before perhaps while a college student or as part of a job. In that case youll need to consider several.
Preparing to apply for a u01 clinical trial registering with clinicaltrialsgov patient research registries clinical trial policies guidelines and templates. In nih strokenet site budget is fixed including overhead and not negotiated at. Phs human subjects and clinical trials information form. Try the clinical e protocol writing tool when youre ready to generate a new protocol using the protocol template.
Put together your own clinical trial budget with this free clinical research budget template. Investigators for such trials are encouraged to use this template when developing protocols for nih funded clinical trials. Nih fda phase 2 and 3 indide clinical trial protocol template. The process however is quite different if you are applying for a grantand could be more challenging if you propose to conduct a clinical trial ct.