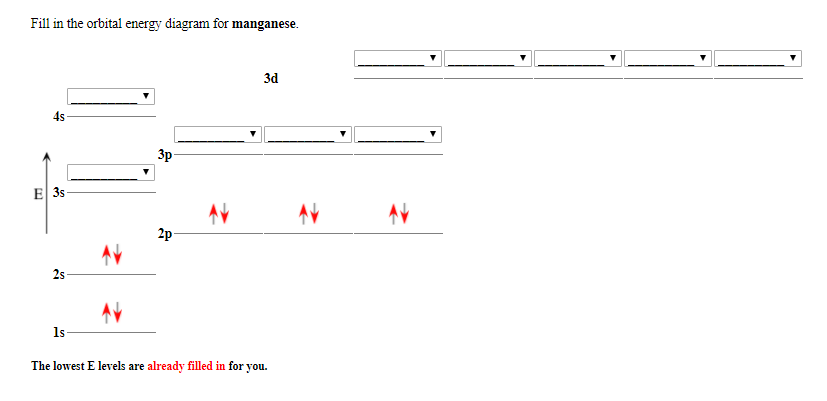
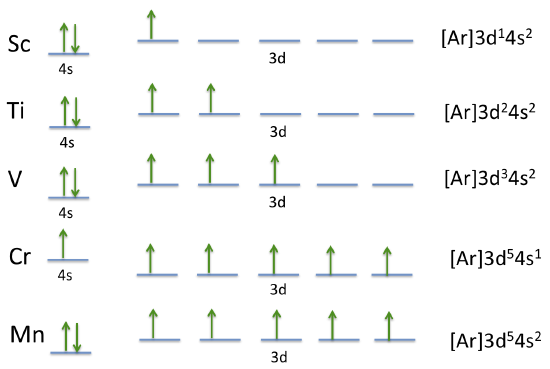
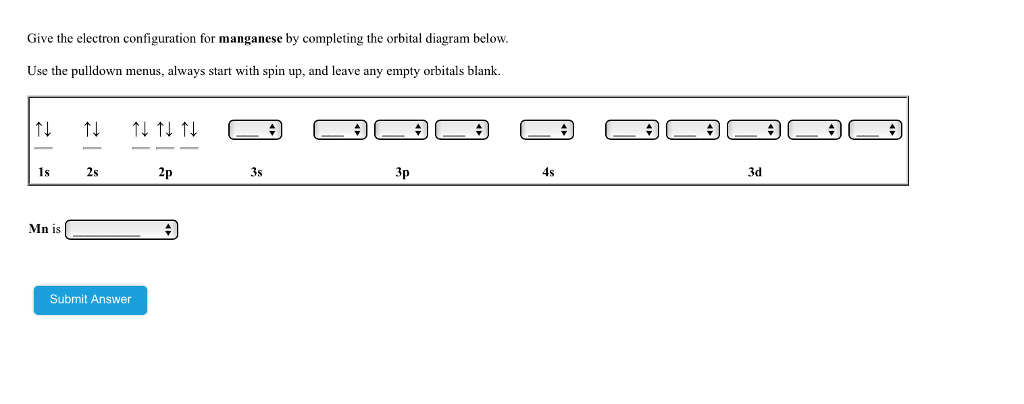
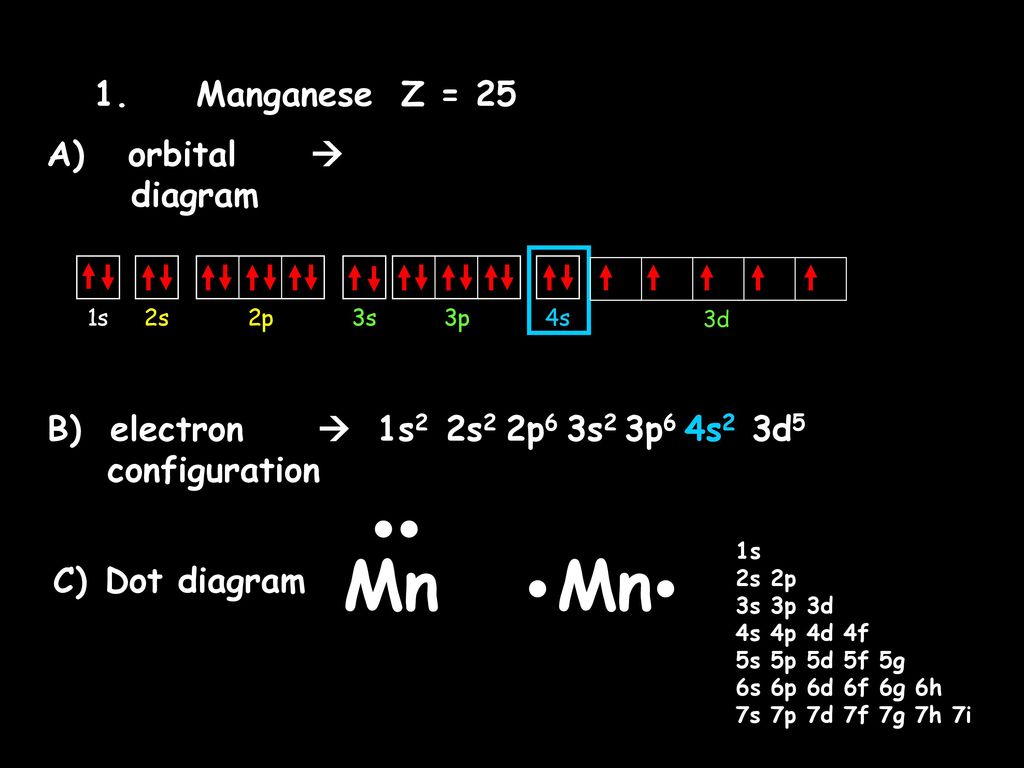
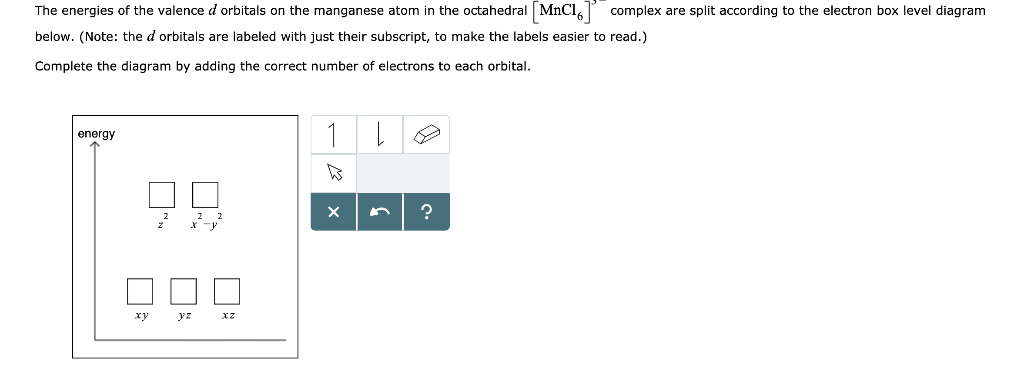
Orbital Energy Diagram For Manganese

Most abundant ores are pyrolusite mno2 psilomelane bah2o2mn5o10 and rhodochrosite mnco3.
Orbital energy diagram for manganese. The three 2p subshells are represented by three dashes of the same energy. This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram notation with arrows of an element. Manganese is another of the many transition elements found.
The 2px orbital lies on the x axis. The orbital diagram for boron as shown has the one electron in the 2p orbital. For the fourth periodrow all of these electrons build the third shell to a maximum of 18 electrons. The fourth row of the periodic table has transition metals ranging from scandium 21 to zinc 30.
Manganese mn chemicalaid manganese mn has an atomic mass of 25 find out about its chemical and physical properties states energy electrons oxidation and more hf molecular orbital diagram orbital diagram for fluorine awesome 0d mos2 2d g. At energy level 2 there are both s and p orbitals with the 2s having lower energy than the 2p. Its also the only orbital in energy level 1. Pure metal produced by mixing mno2 with powered al and ignited in a furnace.
4 partial molecular orbital diagram for plexes 2a 3a and 3a the arrows are intended to highlight the homolumo energy gaps all the dft energy values manganese mn chemicalaid manganese mn has an atomic mass of 25 find out about its chemical and physical properties states energy electrons oxidation and more. Remember that the first eight were placed during our trip through the third periodrow. The energy required to pair the first 2s electron is less than the energy required to place the electron into the 2p orbital. Describe the two differences between a 2p x orbital and a 3p y orbital.
The 1s orbital is closest to the nucleus and it has the lowest energy.