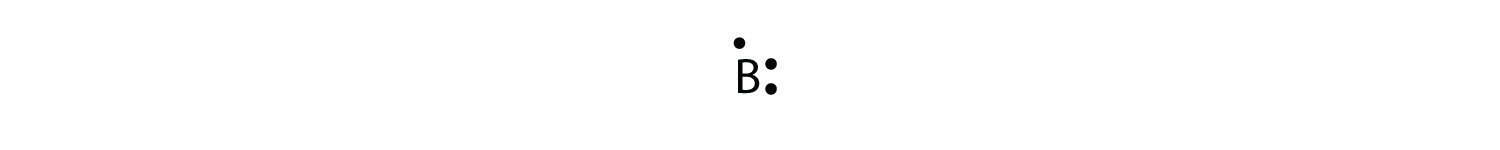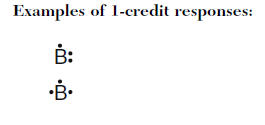Dot Diagram For Boron

3 are in the valence shell.
Dot diagram for boron. A chemistry book perhaps. Mc006 1jpg the shape of an nh3 molecule is. Boron will be at the center of the structure because of being least electronegative. Exercises explain why the first two dots in a lewis electron dot diagram are drawn on the same side of the atomic symbol.
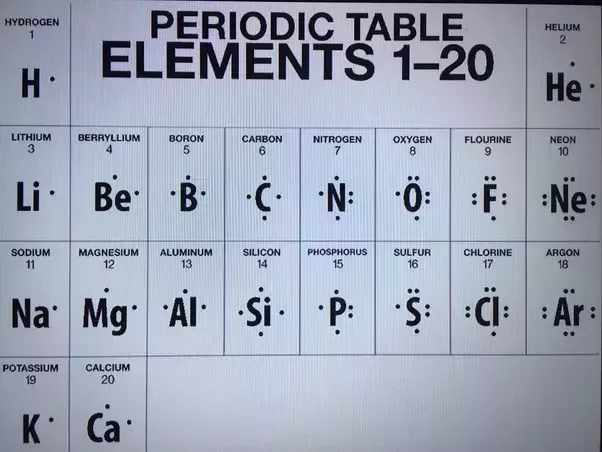
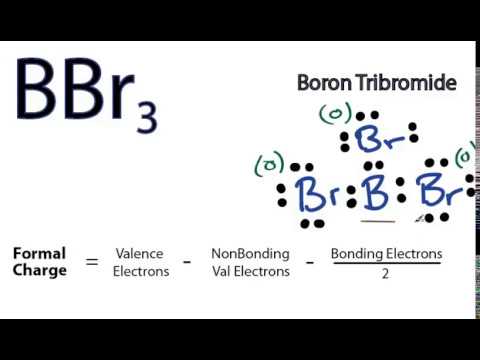
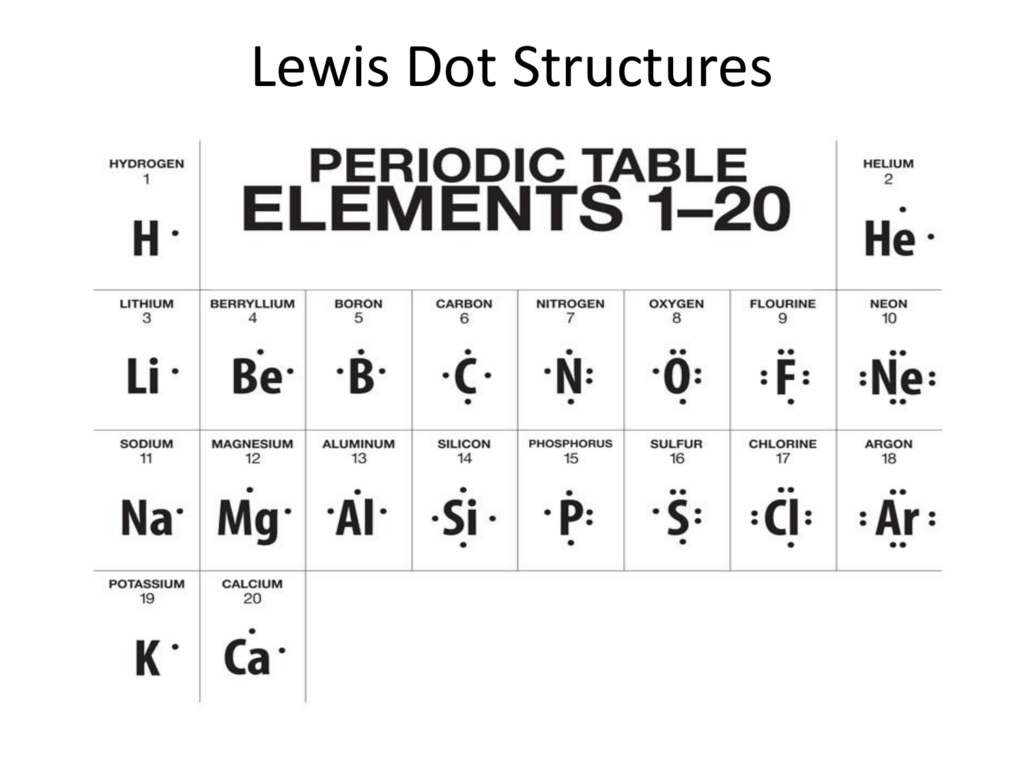
The lewis structure for li is li with one dot to the right of the element. The boron atom of bbr3 has. Here is a table showing the first 20 atoms by atomic number. A step by step explanation of how to draw the lewis dot structure for b boron.
The angle between the hydrogen atoms in water h2o is slightly less than expected for a tetrahedral shape. It requires six valence electrons in its outer shell. Boron is the fifth element with a total of 5 electrons. Bf is unusual in that the dipole moment is inverted with fluorine having a positive charge even though it is the more electronegative element.
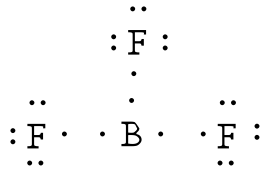
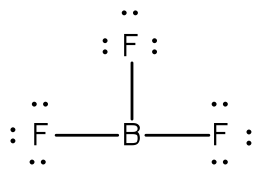
So boron can use those electrons to bond with three other atoms. Boron has 5 electrons. The remaining electron will go in the 2p orbital. If we check the formal charges for the boron trifluoride lewis structure we will find that they are zero even though boron only had six valence electrons.
In writing the electron configuration for boron the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for b goes in the 2s orbital. Lewis dot diagram structures show three formal alternatives for describing bonding in boron monofluoride. I show you where boron is on the periodic table and how to determine how many valence electrons boron has.