Iso 17025 Procedures Templates

We offer a quick documentation kit with ready to use templates to get isoiec 17025 certificate by using our iso 17025 manual procedures forms sop and audit checklist.
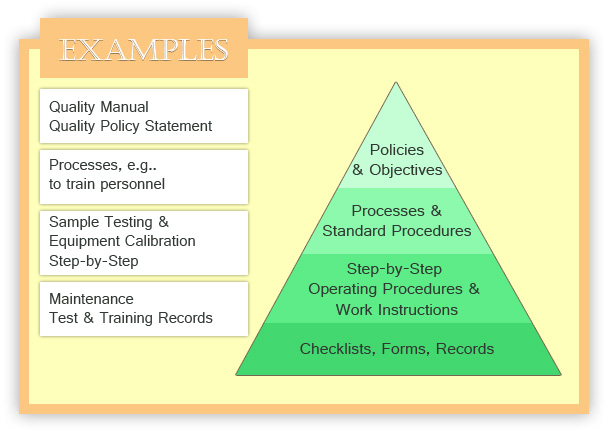
Iso 17025 procedures templates. Quality manual procedures forms the iso 17025 quality manual template is the most efficient approach for you to implement your iso17025 based quality management system. Iso 17025 procedures templates free. Check out the iso 170252017 procedure form free preview. Bundle includes 24 prewritten procedures in microsoft word and help to simplify the implementation of iso 17025.
Iso 170252017 consulting available. The iso 17025 quality manual template allows laboratories to quickly and easily develop or upgrade their quality management system. Develop iso 17025 documentation covering quality manual test instructions work instructions procedures checklists forms and checklist. A news press releases isoiec 170252017 includes many changes.
The iso 170252017 procedure form is included with the following. The need to gain iso 17025 compliance and accreditation impacts many laboratories. There are three main points to keep in mind. Buy the iso 170252017 quality manual template or iso 170252017 management system template that includes the measurement uncertainty calculator forms procedures.
Iso 17025 document template. Built with microsoft office. The iso 17025 quality manual template package includes everything as follows. The iso 17025 procedure bundle provides a starting point for new procedures or serves as a basis for enhancing existing procedures.
Consultants use this package as well. The form style is consistent with the styles used throughout all documents forms and templates. Download the iso 170252017 management system template. The organizations going for iso 17025 accreditation are always in search of readymade documentation to save time.
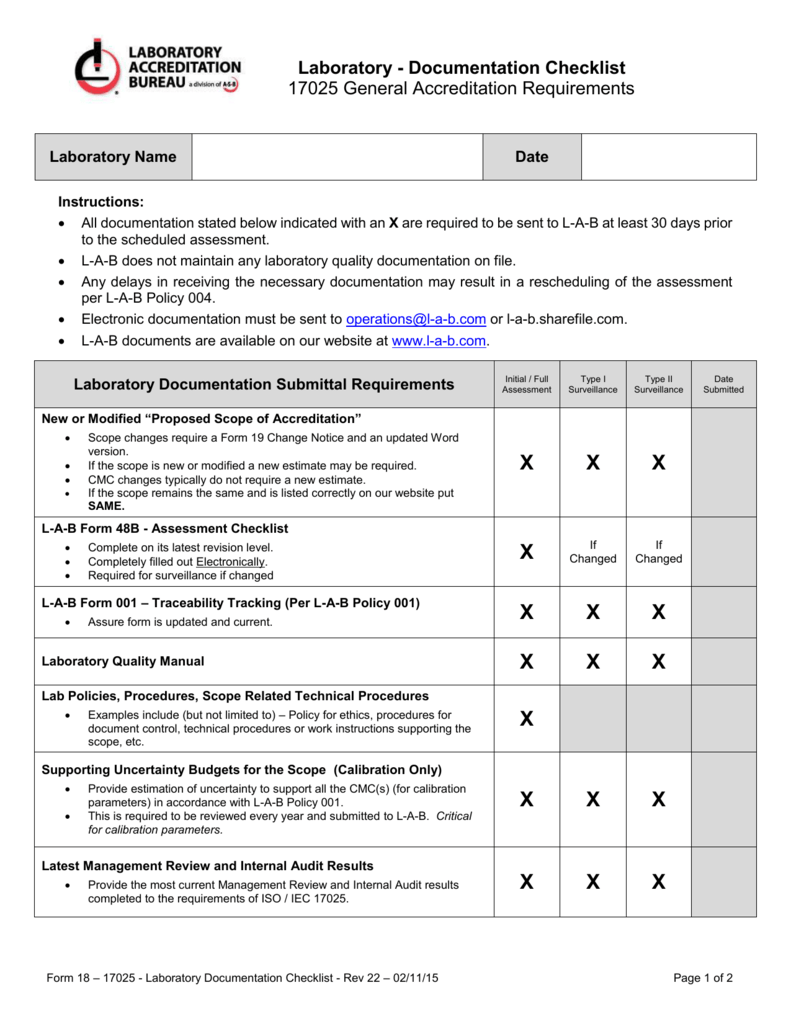
More options involvement of risk updates in current technology. Isoiec 170252017 forms bundle. Document and record control procedure the purpose of this procedure is to ensure control over creation approval distribution usage updates retention and disposition of documents and records also called documented information used in the lms lab management system for performing testing andor calibration laboratory activities. Details about documentation required for iso 17025 compliance iso 17025 accreditation.
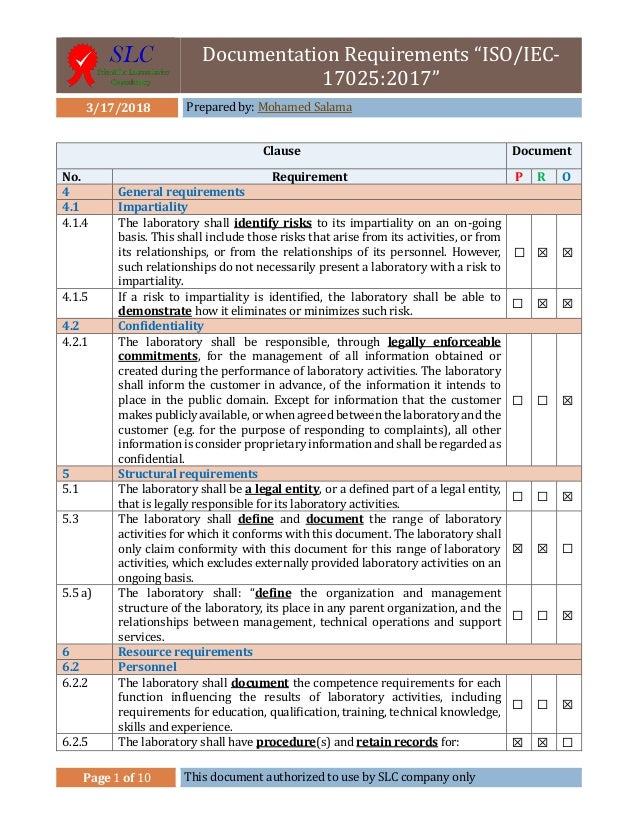
Isoiec 170252017 general requirements for the competence of testing and calibration was released in nov 2017. Laboratories use iso 17025 to implement a quality system aimed at improving their ability to consistently produce valid results. Includes a quality manual 24 procedures 28 forms and 10 lists.