Who Gmp Certificate Renewal
This certificate which is in the format recommended by who establishes the status of the pharmaceutical product and of the applicant for the certificate in the exporting country.
Who gmp certificate renewal. Gmp certificates are issued in a common eu format defined by the european medicines agency ema. The date of the certificate will be the date of issuance. In the past years thousands of gmp and gdp professionals already relied on the programme to advance their knowledge and to get an additional qualification and completed the eca certification level. It is for a single product only since manufacturing arrangements and approved information for different dosage forms and different strengths can vary.
Requirements fees and charges pr application form for the issuance of certificate of accreditation to meat transport vehicles mtv requirements fees and charges mtv application form for the issuance of gmphaccp certificate to meat establishments. For each site one gmp certificate can be issued per domain 1 that has been inspected. The different types of certificates developed by who over the last two decades under the certification scheme as well as examples of other certificates issued by drug regulatory authorities in the exporting countries are described below. One reason for the eca academys excellent reputation is its high quality certification programme.
Good manufacturing practice gmp applicable for all healthcare manufactures such as apis drugs diagnostics food netraceutical medical device cosmetics. A certificate of pharmaceutical product who 1975 type. A gmp certificate is issued to a site and refers to one specific address. Gmp good manufacturing practice certificate.
Respective country guidelines the requirements to be followed for the type of products manufactured based on the impact the quality of a. The eudragmdp database is maintained and operated by the ema. Good manufacturing practice gmp describes the minimum standard that a medicines manufacturer must meet in their production processes. Requirements fees and charges gmphaccp application form for sales promotion permit.
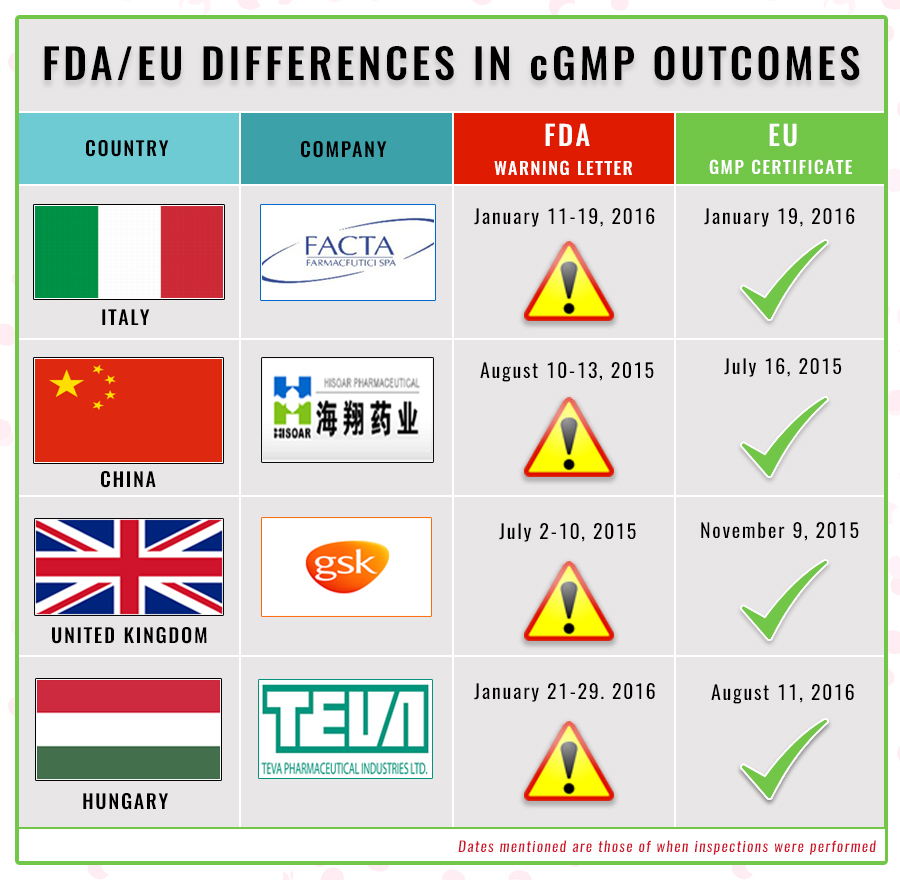
Gmp certification and gmp certificate in europe an overview again and again we receive questions about gmp certification and gmp certificates. Gmp and gdp certification programme. Please upload all copies of information such as license and its renewal approved list of products with its renewal copy site master file who gmp certificate copps process validation reports stability study reports bmrs etc in pdf format only.


.jpg)